X Chromosome Inactivation: A Breakthrough in Genetics
The phenomenon of X chromosome inactivation (XCI) provides a fascinating glimpse into how our genetic makeup influences cellular functions, particularly in females who possess two X chromosomes. This unique biological process ensures that one of the X chromosomes is effectively silenced, preventing an overabundance of gene expression that could lead to complications. Research led by scientists like Jeannie T. Lee has illuminated the molecular mechanisms underlying XCI, revealing the role of gel-like substances in facilitating this crucial chromosomal silencing. By understanding X chromosome inactivation better, researchers are moving closer to potential treatments for genetic disorders such as Fragile X Syndrome and Rett Syndrome. As we delve into the complexities of chromosomal studies, the hope is to unlock new avenues for therapeutic interventions that could transform the lives of those affected by these X-linked conditions.
Chromosomal dosage compensation is a critical process in genetics, particularly pertinent to the mechanisms surrounding X chromosome inactivation (XCI). This process not only regulates gene expression in females but also serves as a focal point of research on various genetic disorders, including Fragile X Syndrome and Rett Syndrome. Pioneering work by researchers, including Jeannie T. Lee, has shed light on how specific genes and cellular behaviors orchestrate this inactivation, offering insights into potential therapies for alleviating the impacts of genetic ailments. By harnessing our understanding of chromosomal dynamics, scientists aim to develop innovative treatment strategies that address the underlying causes of these conditions. Thus, the exploration of XCI continues to be a vital area of investigation within genetic and biomedical research.
Understanding X Chromosome Inactivation: A Scientific Breakthrough
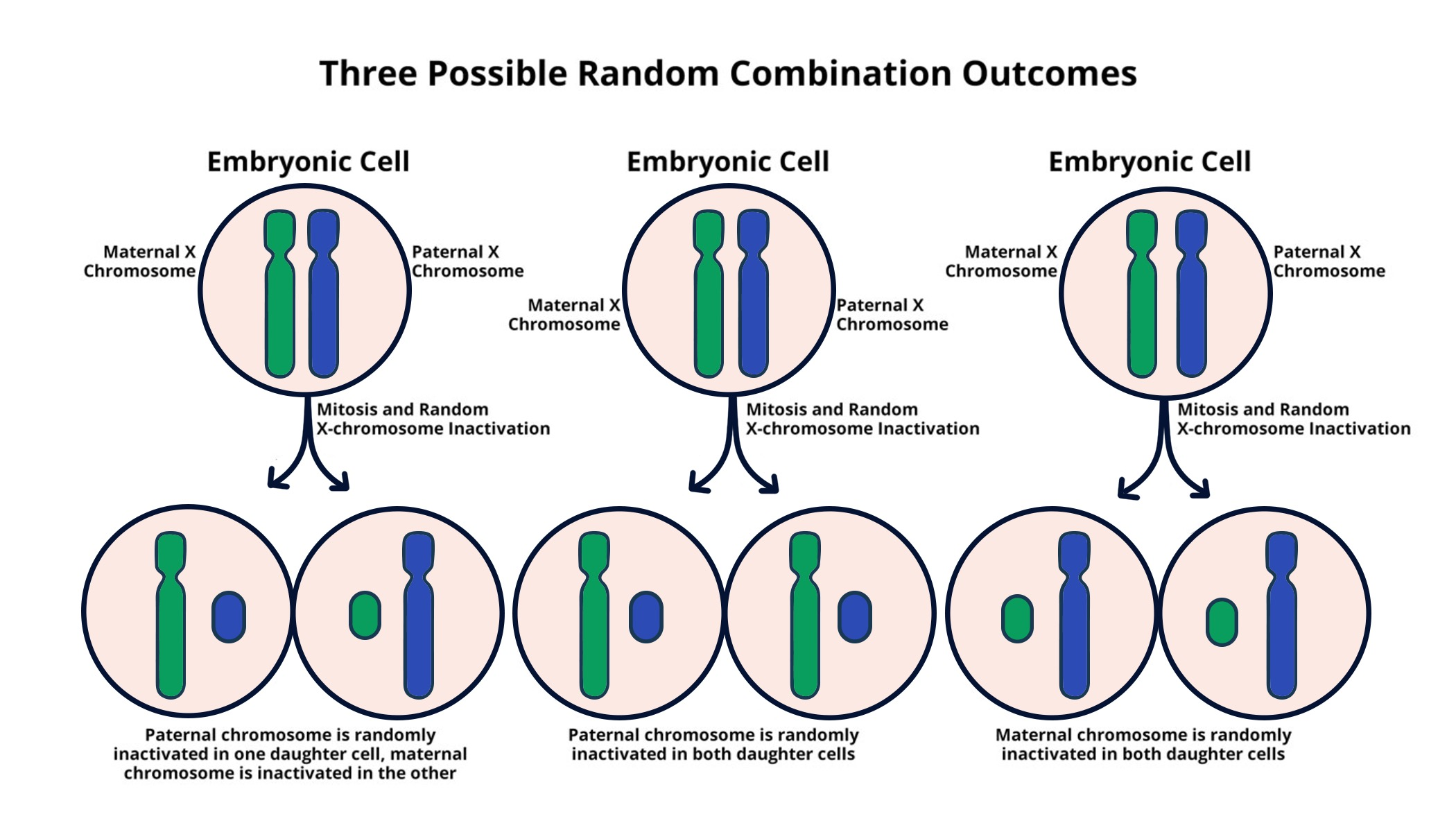
X chromosome inactivation (XCI) is a crucial biological process that occurs in female mammals, where one of the two X chromosomes in each cell is randomly inactivated to ensure dosage compensation between sexes. This phenomenon, first discovered in the 1960s, is essential for preventing the overexpression of X-linked genes in females. Jeannie T. Lee’s research at Harvard Medical School has been pivotal in revealing the mechanisms that underlie XCI. Lee and her colleagues have identified how a gelatinous substance, described as having Jell-O-like properties, plays a key role in the inactivation process, allowing for the efficient silencing of one of the two X chromosomes in females. This understanding not only sheds light on basic cell biology but also opens avenues for potential therapies for various X-linked genetic disorders, including Fragile X Syndrome and Rett Syndrome.
The process of X chromosome inactivation is intricate, involving the engagement of several key molecules, including Xist RNA, which directs the chromosomal silencing. Lee’s lab has noted that when Xist interacts with this gelatinous material, it leads to significant biomechanical changes that facilitate the accessibility of the chromosome for silencing. Such findings are groundbreaking, as they help explain the long-standing question of how and why females can exhibit normal phenotypes despite having two copies of the X chromosome. The implications of understanding XCI extend beyond basic biology, potentially paving the way for innovative treatments that could reactivate genes linked to severe conditions, thus providing hope for individuals afflicted by genetic disorders that stem from mutations on the X chromosome.
The Role of Genetic Disorders in Chromosome Studies
Genetic disorders associated with mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome, provide significant insights into the consequences of chromosomal abnormalities. Fragile X Syndrome, characterized by intellectual disability and behavioral challenges, results from the expansion of a CGG repeat in the FMR1 gene on the X chromosome. On the other hand, Rett Syndrome predominantly affects females and is caused by mutations in the MECP2 gene, also located on the X chromosome. Understanding these disorders has driven researchers like Jeannie T. Lee to explore the mechanisms of X chromosome inactivation (XCI) and its implications for gene expression and disease manifestation. Insights gained from studying these genetic conditions enhance our knowledge of how abnormalities in chromosomal regulation contribute to the clinical features observed in affected individuals.
The interplay between chromosomal studies and genetic disorders highlights the importance of understanding XCI in developing targeted therapies. By comprehending how X-linked genes are silenced or activated, scientists can devise strategies to unsilence these genes in conditions like Fragile X Syndrome and Rett Syndrome. For instance, Lee’s lab is investigating methods to exploit the inactivation mechanism to restore gene function in cells with mutated genes, potentially offering new treatment options. The convergence of chromosomal biology with the study of genetic disorders allows for a more profound exploration of therapeutic avenues that could alleviate the burden of these conditions, reflecting the ongoing commitment to advancing our understanding of genetics through rigorous scientific research.
Exploring the Mechanisms of Fragile X Syndrome and Rett Syndrome
Richarding to the research of Jeannie T. Lee, the molecular mechanisms underlying fragile X syndrome and Rett syndrome underscore the critical role of specific genes located on the X chromosome. Fragile X syndrome is often associated with the mutation of the FMR1 gene, which manifests in various cognitive and behavioral deficits. In contrast, Rett syndrome, primarily affecting females, arises from mutations in the MECP2 gene, leading to profound neurodevelopmental implications. Understanding these mutations is key to unraveling how they disrupt normal neurodevelopmental processes, emphasizing the need for targeted research in chromosomal studies that focus on X-linked genetic disorders. Lee’s investigation into X chromosomal inactivation provides a promising framework to address these underlying genetic anomalies, showing the potential to restore function to compromised genes.
The engagement of X chromosome inactivation in specific genetic disorders presents unique therapeutic opportunities. Lee’s work has delved into manipulating inactivation processes to reactivate silenced genes associated with fragile X and Rett syndromes. The application of chromosomal silencing tactics could allow for the restoration of normal gene expression patterns in patients, offering hope for ameliorating the symptoms of these debilitating conditions. The synergy of chromosomal studies and genetic disorder research not only propels scientific discovery but also highlights opportunities for clinical applications that could transform the future of genetics and patient care.
Jeannie T. Lee: A Leader in Chromosomal Studies
Jeannie T. Lee stands out as a prominent figure in the field of genetics, particularly in her exploration of X chromosome inactivation and its implications for various genetic disorders. As a vice chair in the Department of Genetics at Harvard Medical School, her research focuses on understanding the complexities surrounding gene expression modulation in females, who possess two X chromosomes. Lee’s investigations reveal the dynamic interactions between the XIST RNA and the chromosomal environment, characterized by its unique gelatinous consistency, which is crucial for the inactivation process. This foundational research not only enriches our knowledge of mammalian biology but also connects directly to pressing health issues related to X-linked disorders like Fragile X syndrome and Rett syndrome.
As she continues to lead pivotal studies, Lee emphasizes the potential clinical applications arising from her work on X chromosome inactivation. Recognizing the therapeutic possibilities that stem from these foundational insights, her lab is actively pursuing methods to reactivate silenced X-linked genes. This approach may revolutionize treatment options for patients suffering from genetic disorders by addressing the very root of the issues associated with X chromosomal mutations. Lee’s commitment to advancing scientific knowledge aids the broader understanding of how chromosomal mechanisms can be harnessed for therapeutic benefits, signifying her lasting impact on both research and patient care in the field of genetics.
Jell-O-like Substance: The Key to Chromosomal Function
The recent discoveries surrounding the Jell-O-like substance that envelops chromosomes represent a significant advancement in our understanding of chromosomal functions, particularly in the context of X chromosome inactivation. This viscous gel-like material serves as a critical component that facilitates the silencing of one of the two X chromosomes in female cells. According to Jeannie T. Lee, the interaction between this gelatinous substance and specific molecules, such as the XIST RNA, is essential for managing the complex process of chromosomal inactivation. By creating a flexible environment that prevents entanglement, this Jell-O-like substance enables effective regulation of gene expression on the X chromosome, showcasing a remarkable example of biological innovation.
The significance of this gelatinous material extends beyond mere structural function; it is also a potential avenue for therapeutic intervention. Lee’s research indicates that manipulating the properties of the Jell-O-like substance might lead to breakthroughs in unsilencing genes responsible for Fragile X syndrome and Rett syndrome. By understanding how these substances interact with the XIST RNA and other regulatory elements, researchers can aim to develop treatments that restore normal function to genes that have been rendered inactive due to chromosomal processes. As the field of genetics continues to advance, elucidating the mechanisms behind this unique gel-like substance will be critical for uncovering new strategies to address X-linked genetic disorders.
Future Directions in X Chromosome Research
The trajectory of research on X chromosome inactivation is poised for exciting developments, driven by the foundational work of scientists like Jeannie T. Lee. As researchers continue to understand the nuances of XCI, the focus will increasingly shift towards translating these insights into clinical therapies that address genetic disorders. With ongoing advancements in genetic engineering and cellular manipulation, there is a promising outlook for techniques that could revolutionize how we manage conditions such as Fragile X syndrome and Rett syndrome. By targeting the silenced genes on inactivated X chromosomes, there is potential to significantly improve the quality of life for individuals with these conditions.
Moreover, the implications of this research extend beyond the realms of therapy. It offers a revitalized framework for understanding chromosomal behavior in males and females, providing insights that could impact a broader spectrum of genetic diseases. As Lee’s lab explores innovative strategies to exploit these chromosomal mechanisms, their work will likely lay the groundwork for future discoveries that link X chromosome inactivation to other genetic disorders traditionally not associated with X-linked mutations. The continued exploration of chromosomal studies will unlock new possibilities for precision medicine, empowering scientists to craft specific therapies tailored to the unique genetic landscapes of individuals.
Implications for Genetic Therapy Development
The advancements in understanding X chromosome inactivation have far-reaching implications for the development of genetic therapies. Jeannie T. Lee’s research highlights how manipulating the inactivation process can potentially reactivate silenced genes, thereby offering new hope for patients affected by Fragile X syndrome and Rett syndrome. Approaches that target the Jell-O-like substance surrounding chromosomes, as defined in Lee’s studies, may enable scientists to create interventions that selectively restore the expression of mutated genes. This specificity could minimize unintended side effects, allowing for safe and effective therapeutic options for individuals suffering from these genetic conditions.
Furthermore, the strategies derived from Lee’s findings are expected to influence the broader field of genetic therapy. As the mechanism of X chromosome inactivation becomes better understood, it could serve as a model for addressing other genetic disorders linked to different chromosomal abnormalities. The insights gained from XCI research not only promise breakthroughs in treating neurodevelopmental disorders but may also lead to innovative methods for tackling a wide array of genetic diseases. The convergence of chromosomal studies and therapeutic developments exemplifies the potential for science to address challenging medical conditions through targeted, evidence-based strategies.
The Future of X-linked Disorder Research
Research on X-linked disorders is entering a transformative phase, largely propelled by insights from studies on X chromosome inactivation. The groundbreaking work of Jeannie T. Lee and her collaborators stands at the forefront of this research, offering hope for innovative treatments for conditions such as Fragile X syndrome and Rett syndrome. As our understanding of how XCI operates continues to advance, scientists are poised to explore new therapeutic avenues that leverage this knowledge. This is particularly important given the alarming prevalence of these genetic disorders, which affect thousands of individuals worldwide.
The future of X-linked disorder research looks promising with the potential for developing targeted therapies that could reactivate silenced genes while minimizing side effects. The interplay between basic biological research and clinical applications will be crucial in shaping the next generation of treatments. Within this landscape, Lee’s contributions may serve as a catalyst for discoveries that not only enhance our understanding of X-linked genetic disorders but also ultimately lead to effective strategies for improving the lives of patients affected by these conditions. The journey toward unlocking the secrets of the X chromosome is an invitation for continued exploration and innovation in the field of genetics.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to genetic disorders?
X chromosome inactivation (XCI) is a mechanism in female mammals that randomly silences one of the two copies of the X chromosome to ensure dosage compensation of X-linked genes. This process is crucial for genetic disorders like Fragile X Syndrome and Rett Syndrome, which are caused by mutations on the X chromosome. Through XCI, females typically have one active X chromosome, while males have only one, highlighting the importance of understanding this biological phenomenon for potential therapies.
How does Jeannie T. Lee’s research contribute to our understanding of X chromosome inactivation?
Jeannie T. Lee’s research at Harvard Medical School has been pivotal in uncovering the mechanisms of X chromosome inactivation. Her lab discovered how the RNA molecule Xist interacts with surrounding chromosomal structures to facilitate the silencing of the X chromosome. This breakthrough is especially relevant for targeting genetic disorders like Fragile X Syndrome and Rett Syndrome, as it opens the door for innovative therapeutic approaches to unsilencing mutated genes.
What are the implications of X chromosome inactivation for diseases like Fragile X Syndrome?
X chromosome inactivation has significant implications for diseases such as Fragile X Syndrome, where mutations occur on the X chromosome. The inactivation process can obscure the healthy version of a gene from expression. Understanding XCI can enable researchers to develop treatments that could ‘unsilence’ these genes, providing a potential pathway to alleviate symptoms and improve the lives of those affected by these genetic disorders.
What role does the gelatinous substance play in X chromosome inactivation?
The gelatinous substance surrounding chromosomes, often likened to ‘Jell-O’, plays a critical role in X chromosome inactivation. It provides the structural support necessary for chromosomal organization and facilitates the interaction of molecules like Xist, which modifies this substance to enable the silencing process. This transformation is essential for the proper functioning of XCI and has implications for understanding related genetic disorders.
Can X chromosome inactivation be reversed to treat conditions like Rett Syndrome?
Yes, emerging research suggests that the X chromosome inactivation process can be reversed to treat genetic conditions like Rett Syndrome. By utilizing techniques developed in Jeannie T. Lee’s laboratory, researchers are exploring ways to ‘unsilence’ the mutated genes on the inactivated X chromosome. This approach could lead to novel therapies that restore the function of affected genes, thus providing hope for those with X-linked disorders.
How does X chromosome inactivation differ between males and females?
X chromosome inactivation is a unique process in females, who have two X chromosomes, compared to males, who possess only one. In females, one X chromosome is randomly inactivated in order to equalize gene dosage between the sexes. This process influences how genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome, manifest in individuals of different sexes and highlights the importance of XCI in genetic research and treatment.
What potential therapies are being developed based on findings about X chromosome inactivation?
Researchers, including Jeannie T. Lee, are developing potential therapies that leverage the understanding of X chromosome inactivation to treat disorders like Fragile X Syndrome and Rett Syndrome. These therapies aim to unsilence genes that have been inactivated due to XCI, thereby allowing the healthy gene to express and compensate for mutations. The ongoing research promises to pave the way for innovative clinical trials targeting these genetic disorders.
| Key Point | Details |
|---|---|
| X Chromosome Characteristics | Females have two X chromosomes while males have one. Inactivation is needed to balance gene dosage. |
| Xist Role | A gene on the X chromosome produces an RNA molecule called Xist, critical for the inactivation process. |
| Mechanism of Inactivation | Xist changes the ‘Jell-O’ surrounding the chromosome, allowing other molecules to inactivate the X. |
| Therapeutic Implications | Research may lead to treatments for diseases like Fragile X Syndrome and Rett Syndrome by unsilencing mutated genes. |
| Current Research Status | Continued optimization and safety studies to lead into clinical trials are planned. |
| Future Challenges | Understanding the precise mechanisms of gene utilization post-inactivation remains an important question. |
Summary
X chromosome inactivation is a crucial biological process ensuring that females, with two X chromosomes, do not express twice the amount of X-linked genes compared to males. This intricate mechanism, particularly involving the Xist RNA, paves the way for groundbreaking treatments of genetic disorders linked to the X chromosome. The ongoing research by Jeannie T. Lee and others not only sheds light on how this inactivation occurs but also holds promise for therapies that could alleviate conditions like Fragile X and Rett syndromes. As scientists continue to explore the potential of unsilencing inactivated genes, the future of treating these diseases with minimal side effects emerges as a beacon of hope.